Embark on a journey through the intricacies of solubility curves with our comprehensive guide to solubility curve practice problems worksheet 1 answers. This resource delves into the fundamental concepts, practical applications, and advanced topics surrounding solubility curves, empowering you with a thorough understanding of this essential chemical phenomenon.
Our team of experts has meticulously crafted a set of practice problems ranging from beginner to advanced levels, providing you with ample opportunities to test your knowledge and refine your problem-solving skills. Each problem is accompanied by detailed solutions, ensuring that you grasp the underlying principles and methodologies.
Solubility Curves: Practice Problems and Applications: Solubility Curve Practice Problems Worksheet 1 Answers
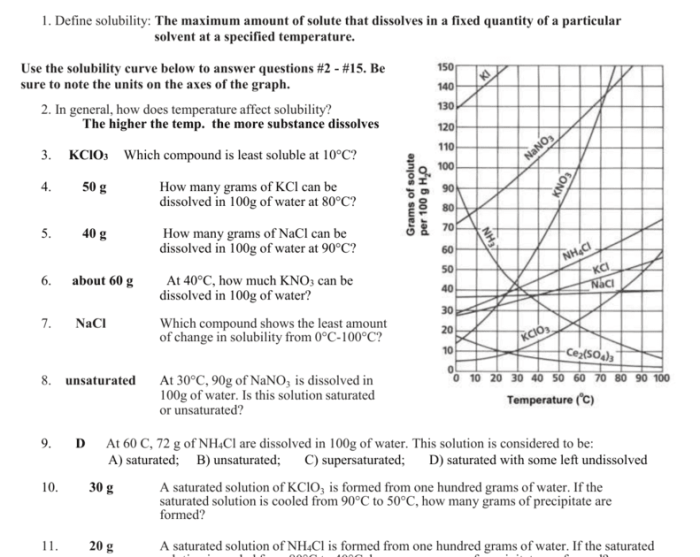
Solubility curves play a crucial role in understanding the behavior of solutions and are widely used in various fields of science and engineering. This worksheet provides practice problems and explores the applications of solubility curves, covering definitions, concepts, and advanced topics.
Definitions and Concepts, Solubility curve practice problems worksheet 1 answers
A solubility curve is a graphical representation of the relationship between the solubility of a substance and the temperature of the solution. It shows how the solubility of a substance changes as the temperature changes.
Solubility varies with temperature in different ways depending on the substance. For most substances, solubility increases with increasing temperature. This is because higher temperatures provide more energy to the solvent molecules, allowing them to break apart the solute molecules and dissolve them more easily.
There are different types of solubility curves, depending on the behavior of the substance in solution. Some common types include:
- Linear solubility curves:The solubility of the substance increases linearly with increasing temperature.
- Curved solubility curves:The solubility of the substance increases at a decreasing rate with increasing temperature.
- Retrograde solubility curves:The solubility of the substance decreases with increasing temperature.
FAQ Resource
What is the definition of a solubility curve?
A solubility curve is a graphical representation that depicts the relationship between the solubility of a substance and temperature or another variable.
How does solubility vary with temperature?
In general, the solubility of most solids in liquids increases with increasing temperature, while the solubility of gases in liquids decreases with increasing temperature.
What are some applications of solubility curves?
Solubility curves find applications in various fields, including predicting the solubility of substances at different temperatures, designing crystallization processes, and understanding the behavior of solutions in different conditions.