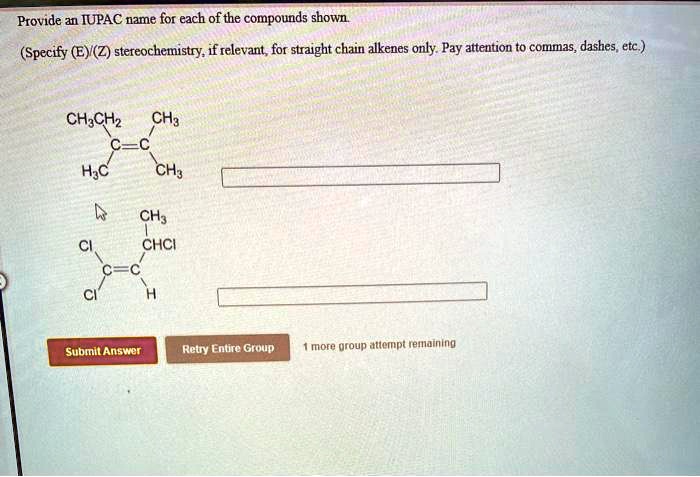
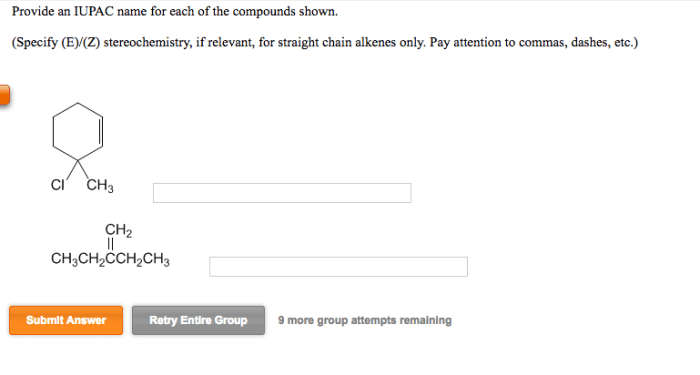
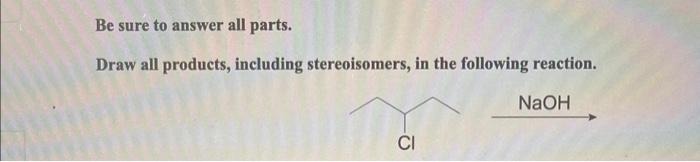
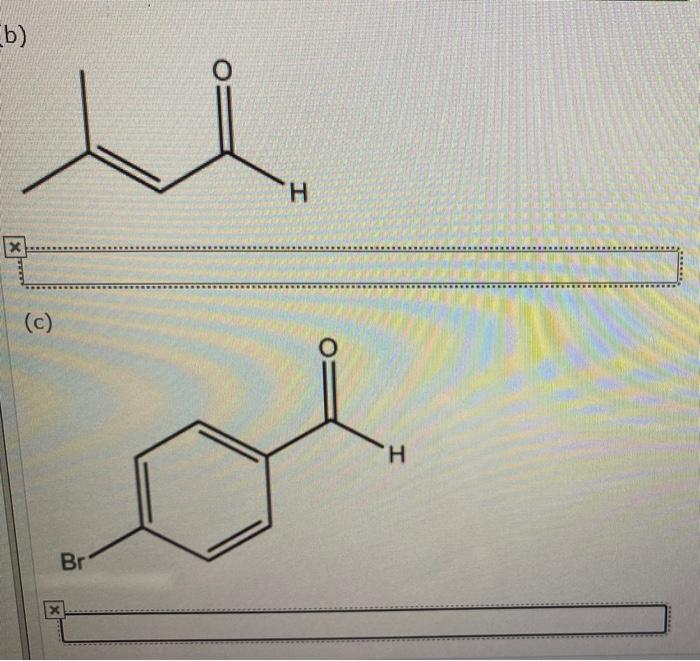
Give the iupac name for each compound. – Give the IUPAC Name for Each Compound: A Comprehensive Guide provides a thorough understanding of the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules. This guide will equip you with the knowledge and skills to accurately name organic compounds, ensuring clear and precise communication within the scientific community.
Delve into the principles of IUPAC nomenclature, exploring the significance of prefixes, suffixes, and locants. Discover how to assign IUPAC names to alkanes, cycloalkanes, alkenes, alkynes, alkyl halides, alcohols, ethers, aldehydes, ketones, carboxylic acids, esters, and amines. Engage with interactive examples and exercises to reinforce your understanding and master the art of IUPAC nomenclature.
IUPAC Nomenclature Principles: Give The Iupac Name For Each Compound.
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming organic compounds to ensure uniformity and clarity in chemical communication. These rules provide guidelines for identifying and naming various functional groups, prefixes, suffixes, and locants to accurately represent the structure of organic molecules.
The IUPAC nomenclature system utilizes prefixes to indicate the number of carbon atoms in the parent chain, suffixes to denote the functional group present, and locants to specify the position of substituents or functional groups along the carbon chain.
Alkanes and Cycloalkanes
Alkanes are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. The IUPAC names for alkanes follow a simple pattern: the prefix indicates the number of carbon atoms in the parent chain, and the suffix “-ane” denotes the alkane functional group.
For example, CH 4is methane, C 2H 6is ethane, and C 3H 8is propane.
Cycloalkanes are cyclic alkanes, meaning they have a ring structure. The IUPAC names for cycloalkanes follow the same principles as alkanes, with the prefix “cyclo” added to indicate the cyclic structure. For example, C 3H 6is cyclopropane, and C 4H 8is cyclobutane.
Alkenes and Alkynes
Alkenes are hydrocarbons that contain one or more double bonds between carbon atoms. The IUPAC names for alkenes follow a similar pattern to alkanes, but the suffix “-ene” is used to indicate the presence of a double bond. The locant is used to specify the position of the double bond, with the lower number indicating the carbon atom with the lower number in the parent chain.
For example, CH 2=CH 2is ethene, and CH 3CH=CH 2is propene.
Alkynes are hydrocarbons that contain one or more triple bonds between carbon atoms. The IUPAC names for alkynes follow the same principles as alkenes, but the suffix “-yne” is used to indicate the presence of a triple bond. The locant is used to specify the position of the triple bond, with the lower number indicating the carbon atom with the lower number in the parent chain.
For example, CH≡CH is ethyne, and CH 3C≡CH is propene.
When multiple double or triple bonds are present, prefixes such as “di,” “tri,” and “tetra” are used to indicate the number of bonds. For example, CH 2=CH-CH=CH 2is 1,3-butadiene, and CH 3C≡C-C≡CH is 1,3-pentadiyne.
Alkyl Halides
Alkyl halides are organic compounds that contain a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to a carbon atom. The IUPAC names for alkyl halides follow the same principles as alkanes, but the suffix “-ide” is used to indicate the presence of the halogen atom.
The locant is used to specify the position of the halogen atom, with the lower number indicating the carbon atom with the lower number in the parent chain. For example, CH 3Cl is chloromethane, and CH 3CH 2Br is bromoethane.
When multiple halogen atoms are present, prefixes such as “di,” “tri,” and “tetra” are used to indicate the number of halogen atoms. For example, CH 2Cl-CH 2Cl is 1,2-dichloroethane, and CH 3CHBr-CH 2Br is 1,2-dibromoethane.
Alcohols and Ethers
Alcohols are organic compounds that contain a hydroxyl group (-OH) bonded to a carbon atom. The IUPAC names for alcohols follow the same principles as alkanes, but the suffix “-ol” is used to indicate the presence of the hydroxyl group.
The locant is used to specify the position of the hydroxyl group, with the lower number indicating the carbon atom with the lower number in the parent chain. For example, CH 3OH is methanol, and CH 3CH 2OH is ethanol.
Ethers are organic compounds that contain an oxygen atom bonded to two carbon atoms. The IUPAC names for ethers follow the same principles as alkanes, but the suffix “-ether” is used to indicate the presence of the oxygen atom. The locants are used to specify the position of the oxygen atom and the two carbon atoms that it is bonded to, with the lower numbers indicating the carbon atoms with the lower numbers in the parent chain.
For example, CH 3-O-CH 3is dimethyl ether, and CH 3CH 2-O-CH 2CH 3is diethyl ether.
Aldehydes and Ketones, Give the iupac name for each compound.
Aldehydes are organic compounds that contain a carbonyl group (C=O) bonded to a hydrogen atom. The IUPAC names for aldehydes follow the same principles as alkanes, but the suffix “-al” is used to indicate the presence of the carbonyl group.
The locant is used to specify the position of the carbonyl group, with the lower number indicating the carbon atom with the lower number in the parent chain. For example, HCHO is methanal, and CH 3CH 2CHO is propanal.
Ketones are organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. The IUPAC names for ketones follow the same principles as alkanes, but the suffix “-one” is used to indicate the presence of the carbonyl group.
The locants are used to specify the position of the carbonyl group and the two carbon atoms that it is bonded to, with the lower numbers indicating the carbon atoms with the lower numbers in the parent chain. For example, CH 3COCH 3is propanone, and CH 3CH 2COCH 2CH 3is pentanone.
Carboxylic Acids and Esters
Carboxylic acids are organic compounds that contain a carboxyl group (-COOH). The IUPAC names for carboxylic acids follow the same principles as alkanes, but the suffix “-oic acid” is used to indicate the presence of the carboxyl group. The locant is used to specify the position of the carboxyl group, with the lower number indicating the carbon atom with the lower number in the parent chain.
For example, HCOOH is methanoic acid, and CH 3CH 2COOH is propanoic acid.
Esters are organic compounds that contain a carboxyl group (-COOR) bonded to an alkyl group (-R). The IUPAC names for esters follow the same principles as carboxylic acids, but the suffix “-oate” is used to indicate the presence of the ester group.
The locant is used to specify the position of the ester group, with the lower number indicating the carbon atom with the lower number in the parent chain. For example, CH 3COOCH 3is methyl acetate, and CH 3CH 2COOCH 2CH 3is ethyl propionate.
Amines
Amines are organic compounds that contain a nitrogen atom bonded to one or more alkyl groups. The IUPAC names for amines follow the same principles as alkanes, but the suffix “-amine” is used to indicate the presence of the nitrogen atom.
The locant is used to specify the position of the nitrogen atom, with the lower number indicating the carbon atom with the lower number in the parent chain. For example, CH 3NH 2is methylamine, and CH 3CH 2NH 2is ethylamine.
When multiple nitrogen atoms are present, prefixes such as “di,” “tri,” and “tetra” are used to indicate the number of nitrogen atoms. For example, CH 3NHCH 3is dimethylamine, and CH 3CH 2NHCH 2CH 3is diethylamine.
Popular Questions
What is IUPAC nomenclature?
IUPAC nomenclature is a standardized system for naming organic compounds developed by the International Union of Pure and Applied Chemistry (IUPAC).
Why is IUPAC nomenclature important?
IUPAC nomenclature ensures consistency and clarity in naming organic compounds, facilitating communication and understanding among scientists.
How do I determine the IUPAC name of an organic compound?
To determine the IUPAC name, you need to identify the parent chain, functional groups, prefixes, and suffixes based on IUPAC guidelines.