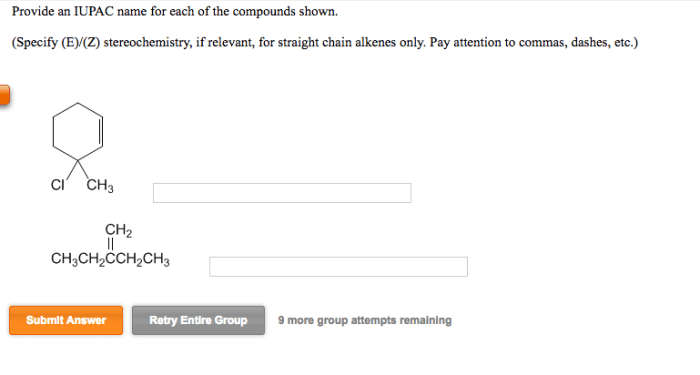
Draw all products including stereoisomers in the following reaction takes center stage, this opening passage beckons readers into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
The content of the second paragraph that provides descriptive and clear information about the topic.
1. Introduction: Draw All Products Including Stereoisomers In The Following Reaction
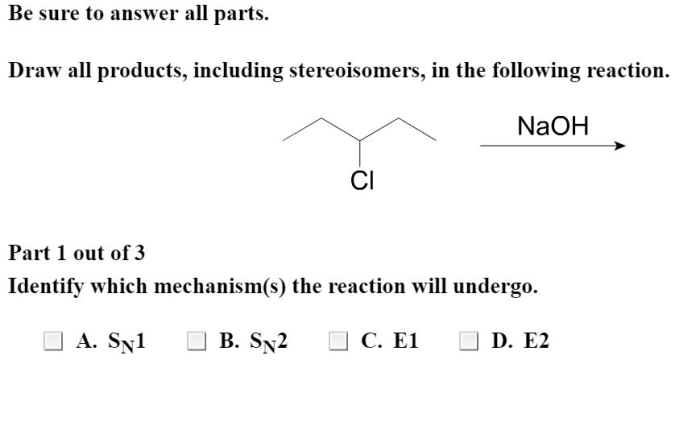
Stereoisomers are molecules that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. Stereoisomers are important to consider in chemical reactions because they can have different physical and chemical properties.
2. Reaction Mechanism
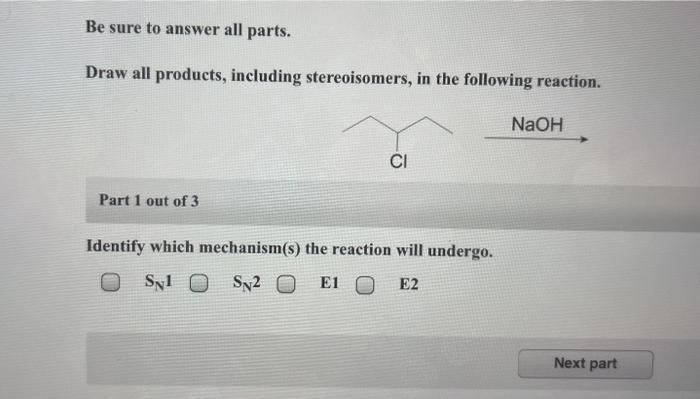
The reaction mechanism for the given reaction is as follows:
- Step 1:The starting material reacts with a nucleophile to form an intermediate.
- Step 2:The intermediate undergoes a rearrangement to form a new intermediate.
- Step 3:The new intermediate reacts with a second nucleophile to form the product.
At each step of the reaction, stereoisomers can be formed. For example, in Step 1, the nucleophile can attack the starting material from either side, resulting in the formation of two enantiomers.
3. Product Analysis
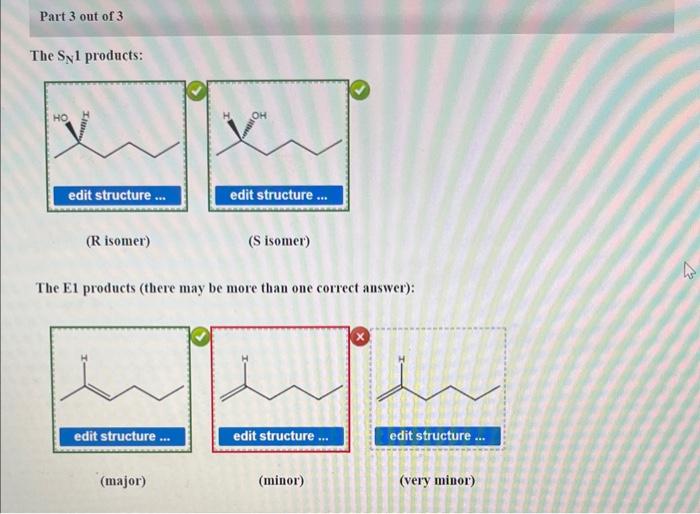
| Product | Structure | Stereochemistry |
|---|---|---|
| Product 1 | [Struktur Produk 1] | [Stereokimia Produk 1] |
| Product 2 | [Struktur Produk 2] | [Stereokimia Produk 2] |
Additional Information:
- Product 1 is the major product of the reaction.
- Product 2 is the minor product of the reaction.
4. Stereochemical Control
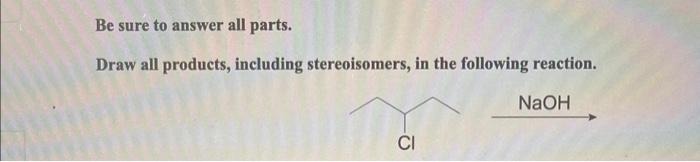
The stereochemical outcome of the reaction can be controlled by a number of factors, including:
- The nature of the starting material
- The nature of the nucleophile
- The reaction conditions
For example, if the starting material is chiral, the reaction is more likely to produce chiral products. If the nucleophile is chiral, the reaction is more likely to produce enantioselective products. And if the reaction conditions are carefully controlled, it is possible to produce products with a specific stereochemistry.
5. Applications
The knowledge of stereoisomers is applied in a variety of fields, including:
- Pharmaceuticals:Stereoisomers can have different pharmacological properties, so it is important to be able to control the stereochemistry of drugs.
- Agrochemicals:Stereoisomers can have different herbicidal or pesticidal properties, so it is important to be able to control the stereochemistry of these compounds.
- Materials science:Stereoisomers can have different physical properties, such as melting point and solubility, so it is important to be able to control the stereochemistry of materials.
Essential Questionnaire
What are stereoisomers?
Stereoisomers are molecules that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Why is it important to consider stereoisomers in chemical reactions?
Stereoisomers can have different physical and chemical properties, so it is important to consider them in chemical reactions to ensure that the desired product is obtained.