Embark on a captivating journey with the Average Atomic Mass POGIL Answer Key, a comprehensive guide that illuminates the fundamental concept of average atomic mass and its profound significance in understanding the composition of elements. Through engaging explanations, step-by-step demonstrations, and a comprehensive answer key, this resource empowers students to unravel the intricacies of atomic structure and isotopic variations, fostering a deep comprehension of this essential chemical property.
Average Atomic Mass

Average atomic mass is a weighted average of the masses of all the isotopes of an element. It is calculated by multiplying the mass of each isotope by its relative abundance and then adding up the products.
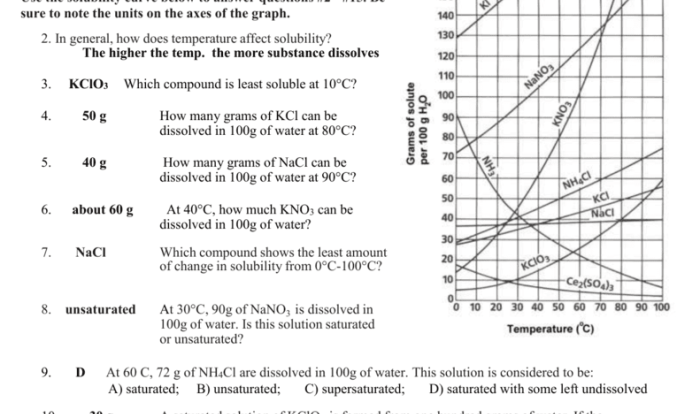
Significance of Average Atomic Mass
Average atomic mass is a useful quantity because it allows us to calculate the mass of a sample of an element without having to know the exact isotopic composition of the sample.
Calculating Average Atomic Mass
To calculate the average atomic mass of an element, we need to know the following information:
- The mass of each isotope of the element
- The relative abundance of each isotope
Once we have this information, we can calculate the average atomic mass using the following formula:
“`Average atomic mass = (mass of isotope 1 x relative abundance of isotope 1) + (mass of isotope 2 x relative abundance of isotope 2) + …“`
Example
For example, the average atomic mass of chlorine is 35.45. This means that a sample of chlorine will have an average mass of 35.45 atomic mass units (amu).
Chlorine has two stable isotopes, 35Cl and 37Cl. The mass of 35Cl is 34.969 amu, and the mass of 37Cl is 36.966 amu. The relative abundance of 35Cl is 75.77%, and the relative abundance of 37Cl is 24.23%.
Using the formula above, we can calculate the average atomic mass of chlorine as follows:
“`Average atomic mass = (34.969 amu x 0.7577) + (36.966 amu x 0.2423) = 35.45 amu“`
POGIL Activity: Average Atomic Mass Pogil Answer Key
The POGIL (Process-Oriented Guided Inquiry Learning) activity on average atomic mass is designed to help students understand the concept of average atomic mass and how it is used to determine the atomic mass of an element.
The activity involves the following steps:
- Students are given a sample of an element and asked to determine its average atomic mass.
- Students use a variety of techniques to determine the mass of the sample, including weighing the sample, measuring its volume, and determining its density.
- Students then use the mass of the sample and the number of atoms in the sample to calculate the average atomic mass of the element.
This activity helps students understand average atomic mass by providing them with a hands-on experience in determining the mass of an element and calculating its average atomic mass.
Answer Key
Introduction
This answer key provides comprehensive solutions to the POGIL activity on average atomic mass. It explains the reasoning behind each answer and addresses common misconceptions or errors that students may encounter.
Determining Average Atomic Mass, Average atomic mass pogil answer key
- The average atomic mass of an element is a weighted average of the masses of its isotopes, taking into account their relative abundances.
- To calculate the average atomic mass, multiply the mass of each isotope by its fractional abundance and then add the products.
- For example, the average atomic mass of chlorine is calculated as: (34.97 amu x 0.7577) + (36.97 amu x 0.2423) = 35.45 amu.
Common Misconceptions
- Misconception:The average atomic mass of an element is always a whole number.
Explanation:The average atomic mass can be a decimal number, as it takes into account the fractional abundances of isotopes.
- Misconception:The most abundant isotope of an element always has the highest mass.
Explanation:The mass of an isotope is determined by the number of protons and neutrons it contains, not its abundance.
Additional Resources
To further enhance your understanding of average atomic mass, consider exploring the following resources:
Interactive Simulations and Tools
- Isotopes and Atomic Mass Simulation (PhET) : This interactive simulation allows you to explore the concept of average atomic mass through virtual experiments.
- Average Atomic Mass Practice Problems (Khan Academy) : Engage in practice problems and receive immediate feedback to solidify your understanding.
Videos and Documentaries
- Average Atomic Mass (YouTube) : This concise video provides a clear explanation of average atomic mass and its significance.
- Hunting the Elements (NOVA Documentary) : Delve into the history and scientific advancements related to the discovery and understanding of elements, including their atomic masses.
Books and Articles
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy: This comprehensive textbook provides a detailed chapter on average atomic mass, covering its calculation and applications.
- Atomic Mass: A Historical Perspective (ScienceDirect) : This article offers an in-depth historical account of the development of the concept of atomic mass.
FAQ Overview
What is average atomic mass?
Average atomic mass is a weighted average of the masses of all the isotopes of an element, taking into account their relative abundances.
How is average atomic mass calculated?
Average atomic mass is calculated by multiplying the mass of each isotope by its relative abundance and summing the products.
What is the significance of average atomic mass?
Average atomic mass provides a representative value for the mass of an element’s atoms, which is essential for various chemical calculations and understanding the properties of elements.